AxonalPlasticityLab@AxonalLab
Sep 23, 2022
9 tweets
So this happened ... our journey into neuronal structure-function relationships is out (but not finished) @Science Magazine! Goes to show what great collaborations can achieve @Both Lab @Burgalossi Lab @Draguhn Lab More to follow in the next tweets ...
science.org/doi/10.1126/sc
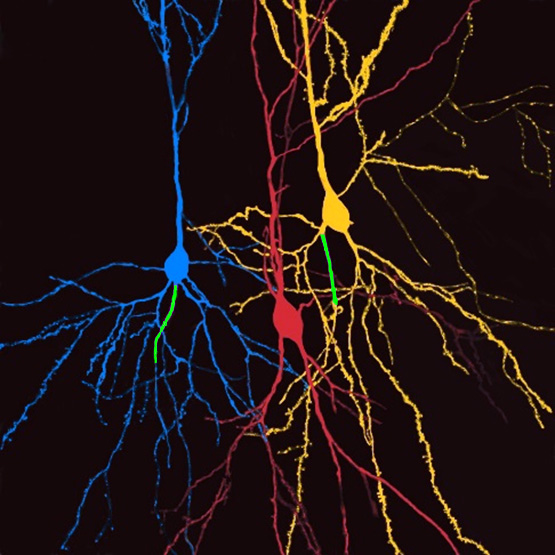
In 2014, we showed that up to 50% of HC pyramids have an axon emerging from a basal dendrite (Thome et al., Neuron). So what is new? Excitatory inputs to this privileged dendrite evade perisomatic inhibition and can generate APs when all other inputs are shunted.
So there you go - perisomatic inhibition is not simply a ‘gain control’ but ‘gates’ information flow in "axon-carrying dendrite cells", or AcD cells. How does that work?
Classic perisomatic inhibition? Rhythmic activity of perisomatically targeting inhibitory neurons plays a key role in oscillations. They synchronously inhibit the soma of pyramids where excitatory inputs are integrated. Thus, they define rhythmic ‘windows of opportunity’ for APs.
Meet @Both Lab 's special neurons: Inhibition is low, therefore we find ‘high gain’, thus easy generation of action potentials.
Vice versa: Inhibition is high = ‘low gain’, no action potentials possible.
So what is different in AcD cells? The basal dendrite that carries the axon effectively evades perisomatic inhibition, and thus the time window for AP generation becomes much larger. The consequence? Inhibition low: ‘high gain’, easy generation of APs.
Inhibition high: ‘low gain’. But outside the time window set by inhibitory gain control, inputs to the specialized dendrite are still able to elicit APs. Can inhibition induce rapid switches between two different functional networks?
We think so! One network configuration with all cells and one exclusively composed of AcD cells. Simply put, perisomatic inhibition closes a gate for input coming from ‘normal’ dendrites such that only the AcD remains functionally relevant. Of course this will not be the end 


AxonalPlasticityLab
@AxonalLab
The Axonal Plasticity Lab is located at the Institute of Anatomy and Cell Biology at JKU Linz, Austria.
Missing some tweets in this thread? Or failed to load images or videos? You can try to .